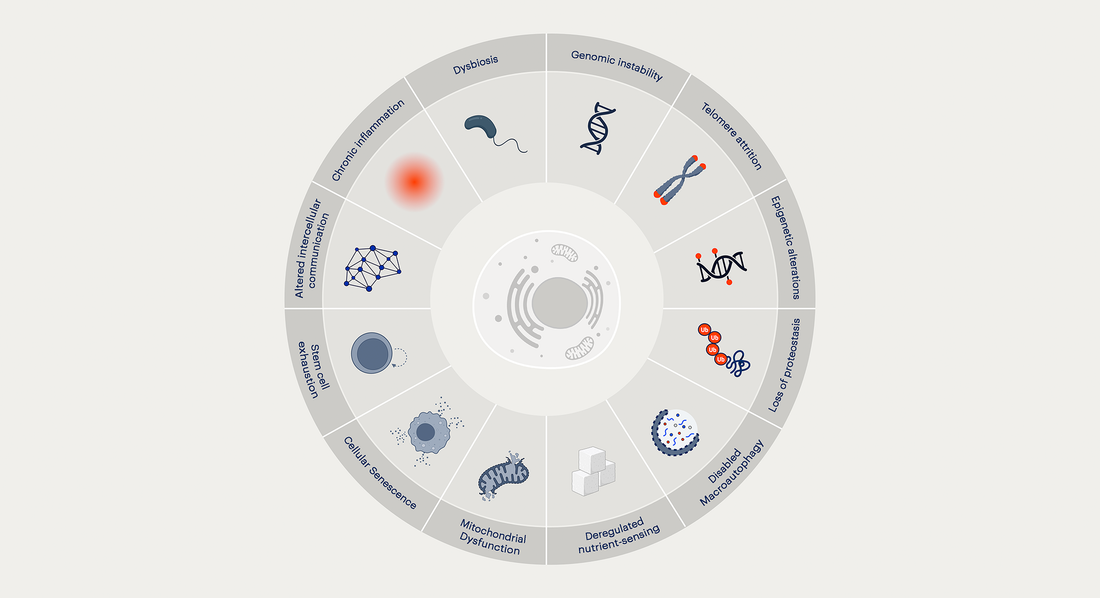
Key Takeaways:
- The Hallmarks of Aging represent 12 interconnected cellular processes that drive aging—ranging from genomic instability to age-related inflammation.
- This framework was derived from decades of research and provides a roadmap for developing interventions that support healthy aging.
- In this article, we break down each hallmark and explore how science-backed ingredients like NAD+ precursors and senolytics target these hallmarks.
Related Products:
Signal and Basis support multiple Hallmarks, including genomic instability and epigenetic alterations by supporting DNA maintenance, while Format addresses age-related inflammation, and the Senolytic Complex targets cellular senescence.
Elysium co-founder and chief scientist Leonard Guarente, Ph.D., is considered “a grandfather of the booming longevity movement,” according to a recent article by Amy Dockser Marcus in The Wall Street Journal (WSJ)—having pioneered the field of aging research more than 30 years ago in his lab at Massachusetts Institute of Technology. Dr. Guarente is “an architect of one of the field’s biggest ideas, has trained its most influential scientists,” writes Marcus and “the field he shaped” has inspired decades of scientific exploration and hundreds of thousands of academic articles. The discoveries in this field were collated into a comprehensive description of the biological process of aging in the 2013 landmark paper published in Cell that formally defined the "Hallmarks of Aging.” This framework was updated in 2023 to encompass 12 interconnected biological processes that drive aging and disease.2 Since its introduction, the Hallmarks of Aging have been cited over 11,000 times, fueling an explosion of research into the mechanisms of aging and potential interventions.
This article provides a high-level overview of each of the 12 Hallmarks of Aging. While summarized concisely, it’s important to recognize that each hallmark represents a vast and intricate field of study, with entire research labs dedicated to unraveling its complexities. For those interested in a more detailed exploration, the updated Hallmarks of Aging paper can be accessed here.
Genomic Instability
The genome (made up of long strings of DNA) serves as the body's blueprint, encoding all the instructions needed for cellular function. It is tightly packed into higher-organizational structures called chromatin and regulated by proteins such as histones that protect DNA from damage while ensuring the right genes are activated at the right time. However, over time, the genome is continually exposed to threats—sun exposure, oxidative stress, and replication errors—that compromise its integrity.
To counteract this, the body relies on a sophisticated network of DNA maintenance mechanisms. However, as we age, these systems become less efficient, increasing the risk of genomic instability, which can contribute to a range of health conditions. NAD+ precursors, such as nicotinamide mononucleotide (NMN) in Signal and nicotinamide riboside (NR) in Basis, help support these maintenance pathways, by increasing NAD+ levels. NAD+ has a cascade of effects that enhance the body's ability to maintain genomic stability (e.g., directly activating cell repair genes, reducing oxidative stress, etc.) Additionally, pterostilbene, found in Basis, has been shown to increase the expression of a group of proteins called sirtuins that help support genomic health. They can facilitate DNA maintenance, stabilize chromatin, regulate gene expression to minimize inflammation, and require NAD+ to function.
Telomere Attrition
Telomeres act as protective caps at the ends of chromosomes, preventing DNA from unraveling during cell division. However, due to limitations in DNA replication, telomeres gradually shorten with each cell division. Over time, critically short telomeres trigger cellular senescence (a dysfunctional dormant state) or apoptosis (self-destruction), limiting tissue regeneration.
Telomere shortening is associated with conditions such as pulmonary fibrosis and aplastic anemia. While the enzyme telomerase can extend telomeres, most differentiated human cells do not naturally produce it, making telomere maintenance a key focus of aging research.
Epigenetic Alterations
The sequencing of the human genome once promised a complete understanding of biology, but scientists have since recognized that genes are only part of the equation. Epigenetic modifications—chemical tags on DNA and its associated proteins—regulate gene activity without altering the genetic code itself. These modifications influence cellular identity and function but are susceptible to age-related disruptions.
NAD+-dependent sirtuin proteins and compounds like pterostilbene (found in Basis) play a role in maintaining epigenetic stability. By supporting proper histone functioning and DNA maintenance, they help sustain cellular health over time.
Loss of Proteostasis
Proteins are the molecular building blocks of the cell, but they must be properly folded and maintained to function correctly. The body's quality control mechanisms detect and degrade misfolded or damaged proteins, preventing cellular dysfunction. However, with aging, these systems become impaired, leading to the accumulation of toxic protein aggregates which might contribute to neurodegenerative conditions.
Sirtuin activation helps support proteostasis. For instance, sirtuin activation helps promote autophagy. This is the process the body uses to identify misfolded proteins and eliminate them. High homocysteine levels have also been shown to interfere with healthy protein maintenance. Matter contains a specific B-vitamin complex that lowers homocysteine levels. Lower homocysteine levels likely enhance protein recycling and promote brain health.
Impaired Macroautophagy
Autophagy is the body's internal recycling program, allowing cells to break down and repurpose damaged components. Macroautophagy, in particular, enables the degradation of entire organelles, such as malfunctioning mitochondria. When autophagy declines with age, cellular waste accumulates, accelerating aging and contributing to diseases like cardiovascular and neurodegenerative disorders.
Researchers continue to explore ways to enhance autophagy, and some products such as spermidine show promise. The morbidity of numerous diseases of aging could be eased with enhanced autophagy.
Deregulated Nutrient Sensing
Cells continuously monitor and respond to their environment through nutrient-sensing pathways. One key regulator, the mTOR pathway, influences cellular growth and aging. Research in animal models has shown that calorie restriction can extend lifespan by modulating mTOR activity, highlighting its role in longevity.
Sirtuin activation has been shown to optimize mTOR signaling, helping to fine-tune the balance between growth and longevity-promoting processes.
Mitochondrial Dysfunction
Mitochondria are truly fascinating organelles, often referred to as the powerhouses of the cell. They generate the energy necessary for cellular function and even have their own DNA. However, mitochondrial DNA (mtDNA) is not as well protected as nuclear DNA, making it more susceptible to irregularities over time. This accumulated effect impacts mitochondrial dysfunction, which can be detrimental to cellular health. In some cases, irregular mitochondria proliferate uncontrollably, suffocating healthy cells.
Affected mitochondria also generate increased oxidative stress, which accelerates aging throughout the cell. Sirtuin activation has been shown to promote mitochondrial biogenesis, potentially reducing some of these effects. Intracellular NAD+ along with surtuin proteins SIRT1 and SIRT3 all have been shown to support mitochondrial health, function, and biogenesis. Basis and Signal both contribute to healthy mitochondria given their NAD+ precursor ingredients and sirtuin activating compounds. Additionally, a mitochondrial-derived peptide called humanin has been identified as a protective factor for mitochondrial health. Research suggests that centenarians exhibit higher humanin levels, which may contribute to their longevity by reducing age-related mitochondrial decline.
Cellular Senescence
Cellular senescence is intricately linked to several other Hallmarks of Aging. As cells accumulate changes over time, they may reach a point where they can no longer divide. Rather than undergoing programmed cell death, these cells enter a state of senescence. Ideally, the body would efficiently detect and clear these changed cells, allowing healthy new cells to replace them. However, with aging, senescent cells accumulate, and the body's ability to eliminate them declines.
Senescent cells not only lose their functional capacity but also contribute to inflammation. They release pro-inflammatory factors, creating a phenomenon known as the senescence-associated secretory phenotype (SASP). Senolytics are compounds that help clear these cells from the body, thereby mitigating inflammation and slowing cellular aging. Elysium’s Senolytic Complex is designed to support the clearance of these cells. Additionally Format contains an inflammaging complex and daily immune support to keep immune cells healthy (immune cells are what clear the body of senescent cells). The Senolytic Complex and Format work together to help the immune system effectively clear senescent cells while reducing inflammaging.
Stem Cell Exhaustion
Stem cells are unique in their ability to divide and generate new cells, playing a critical role in tissue maintenance and repair. Unlike fully differentiated cells, which have a limited number of divisions (a concept known as the Hayflick Limit), stem cells utilize telomerase to enable continuous replication. However, over time, excessive divisions and accumulated damage impair their regenerative capacity.
As stem cell function declines, tissues become less capable of replenishing themselves, leading to age-related degeneration. Researchers are exploring the potential of transcription factors to reprogram differentiated cells into stem-like cells, potentially restoring regenerative function. Such advancements hold promise for reversing aspects of aging by replenishing lost cellular populations.
Altered Intercellular Communication
Effective cellular communication is essential for maintaining homeostasis. In youthful tissues, cells respond efficiently to hormonal and molecular signals, facilitating coordinated function. However, with age, these communication networks degrade, leading to systemic dysfunction.
One well-known example is insulin resistance. As cells become less responsive to insulin, blood sugar regulation is impaired, accelerating metabolic dysfunction and aging. Insulin resistance leads to higher blood sugar levels, which in turn can be damaging to the body in myriad different ways. Insulin dysfunction is one of many different intercellular communication pathways that can be disrupted in aging, which can in turn lead to age-related problems.
Chronic Inflammation
Chronic inflammation is a pervasive consequence of aging, often described as "inflammaging." Virtually every Hallmark of Aging contributes to increased inflammation, which in turn exacerbates age-related diseases such as cardiovascular disease, neurodegeneration, and metabolic disorders.
Inflammation is particularly problematic because it accelerates the breakdown of immune surveillance, allowing damaged or potentially cancerous cells to persist. Furthermore, chronic inflammation is immunosuppressive, impairing the body’s ability to eliminate senescent cells—which are themselves pro-inflammatory (see “Cellular Senescence” above). NAD+ depletion is associated with impaired inflammatory responses and restoring NAD+ levels can help boost adaptive immunity through supporting the health of T and B cells and non-immune cells, and prevent their senescence.
Dysbiosis
Dysbiosis—an imbalance in the gut microbiome—is an emerging area of research with profound implications for aging. Unlike the other Hallmarks of Aging, which primarily concern human cellular function, dysbiosis highlights the critical role of microbial symbiosis in overall health.
A healthy gut microbiome supports digestion, nutrient absorption, immune function, and even neurotransmitter production. However, with age, microbiome diversity declines, leading to increased inflammation, metabolic dysfunction, and susceptibility to disease. Dysbiosis has been linked to obesity, diabetes, neurological disorders, and systemic inflammation—conditions that accelerate aging.
Maintaining a balanced gut microbiome through diet, probiotics, and targeted supplementation can support healthy aging. By nurturing these microbial partners, we enhance not only digestion but also systemic resilience against age-related decline.
This is just an introduction to the Hallmarks of Aging. Each hallmark represents a crucial piece of the aging puzzle, and interventions targeting these pathways hold promise for improving healthspan and longevity. Ongoing research and innovative nutraceuticals, like those developed at Elysium Health, aim to translate this science into practical solutions for healthier aging.
REFERENCES
1. López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
2. López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243–278. doi:10.1016/j.cell.2022.11.001