Aging 101
Explore the Science Behind Our Products.
Your source for information about NAD+, sirtuins, and other important topics in aging science.
-
 Read More
Read More10 years. 10 studies. The science that shaped l...
For the past decade, longevity science has moved from bold hypotheses to concrete human evidence. The Abstract is where we track that shift—documenting the studies that changed how we think about aging. To...
-
 Read More
Read MoreGlossary of scientific terms
A collection of terms commonly used in aging research.
-
 Read More
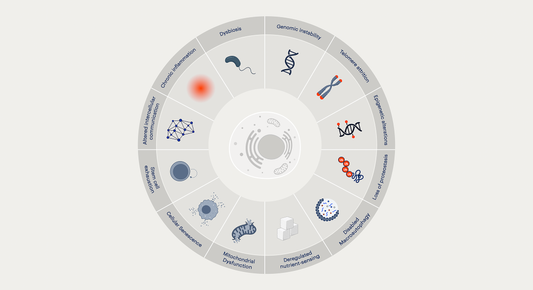
Read MoreWhat are the Hallmarks of Aging?
Discover the 12 Hallmarks of Aging and how ingredients like NAD+ precursors, senolytics, and other targeted compounds address these hallmarks to support healthy aging.
-
 Read More
Read MoreHow does collagen help with joint and bone health?
Collagen loss with age can affect joints and bones. Learn how collagen peptides support mobility, flexibility, and bone strength.
-
 Read More
Read MoreNAD+ injections explained: Why experts recommen...
NAD+ is being offered in various forms including subcutaneous (SC), intramuscular (IM), and intravenous (IV) injections. But do they work? We examine the research behind direct NAD+ administration methods to...
-
 Read More
Read MoreHere's what happens when you take Cofactor, sta...
Explore the benefits of Elysium’s advanced collagen support system, Cofactor, from three weeks to one year and beyond.






